Research Disadvantaged Diseases Create Research Engines Using Patient Registries
Chronic Lyme disease is a research disadvantaged disease. Compared to other diseases very little clinical research has been conducted—particularly for the treatment of chronic Lyme disease where patients remain ill for six or more months following a short course of antibiotics. The National Institute of Health has funded just three clinical trial grants for the treatment of chronic Lyme disease.
Although Lyme disease is estimated to have over 400,000 cases per year, data from clinicaltrial.gov indicates that research for Lyme disease trails behind leprosy, which has an incidence of less than 200 cases a year (Goswami 2013). To date, pharma has shown no interest in developing new treatment drugs for chronic Lyme disease.
This means that even though it is not a rare disease, Lyme disease faces the same research challenges that rare diseases encounter. One way research disadvantaged diseases address their unmet needs is to build a research engine that facilitates and accelerates the pace of research by linking patient registries, biorepositories, and clinical data networks (Groft 2014). The NIH and the Patient Centered Research Outcomes Institute (PCORI) as well as the Agency for Healthcare Research and Quality have led efforts in this area. Dr. Francis Collins recently acknowledged the vital role that the patient led research in characterizing long haul COVID-19 (NIH Directors Blog 2020). Research in chronic Lyme disease needs to move forward at a much more rapid pace than it has historically. To do this it needs to take advantage of all forms of research, including observational research using patient registries.
The MyLymeData Patient Registry and Research Platform
In 2015, LymeDisease.org (LDo) launched the first national patient registry and research platform for Lyme disease, MyLymeData, which was developed as part of PCORnet, PCORI’s patient-driven research effort, when I served on its Executive Committee. I continue to serve as a subject matter expert in patient registries for PCORnet registries through the University of Chicago.
MyLymeData has enrolled over 14,000 patients, collected over 5 million data points, published five peer-reviewed studies, and seven white papers and scientific posters (Johnson 2011, 2014, 2016, 2018, 2019, 2020). It has been highlighted in two chapters in college text books and three government white papers on patient registries (Johnson 2019, Peay 2020). We collaborate with academic researchers at UCLA and the University of Washington as well as the Lyme Biobank, a project of the Bay Area Lyme Foundation. We just published our fifth big data study with UCLA researchers who received a National Science Foundation grant to explore artificial intelligence techniques using data from the registry.
All Forms of Evidence Are Essential and Each has Unique Advantages and Limitations
When the optimal treatment, duration, or combination of treatments is unknown, as it is in Lyme disease, the process of conducting back-to-back sequential randomized controlled trials to determine the best treatment approach is not realistic (Savor 2001). To accelerate the slow pace of research and to provide real-world evidence, observational data like those collected in patient registries hold enormous appeal for the type of detailed analysis essential to precision medicine (Kent 2018).
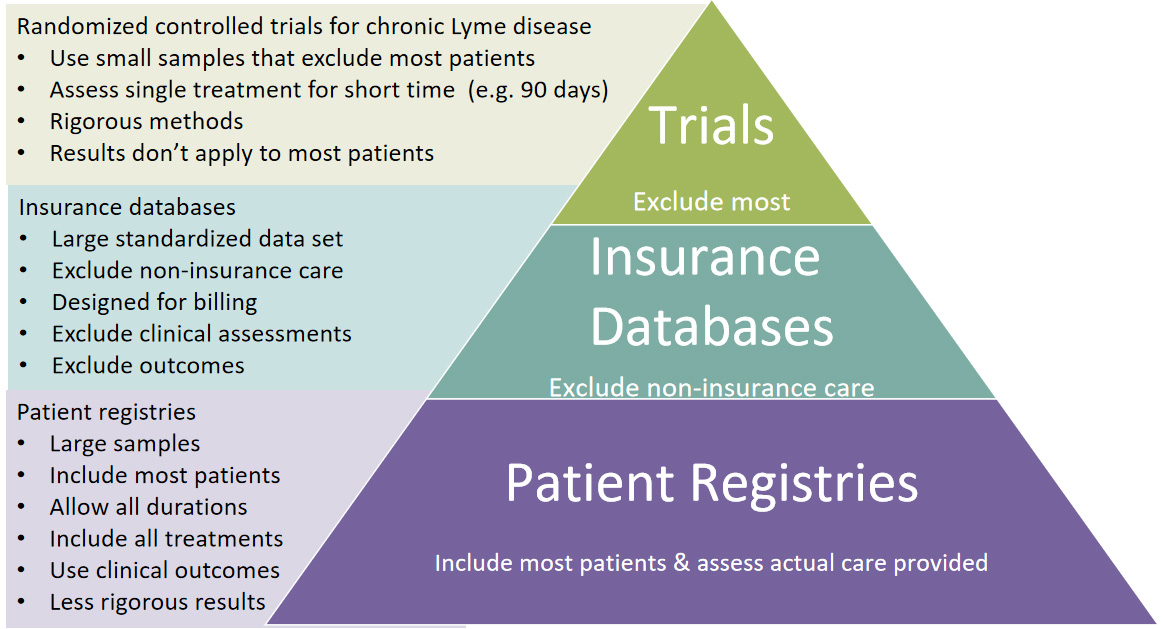
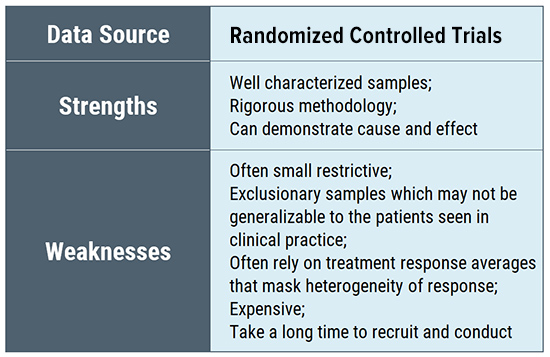
All data sources have their strengths and limitations depending on their source and characteristics. For example, randomized controlled trials are uniquely valued because of their ability to determine cause and effect, but they do so in a process that is inherently time intensive and expensive, typically measuring the effect of a single intervention at a time and generating knowledge sequentially. As one researcher noted:
“It can take more than a decade for a trial to progress from the idea stage to actionable information, and cost and complexity mean that many questions will never be addressed with such trials” (Howie 2014)
In addition, small exclusionary RCT trials, such as the NIH CLD treatment trials, are not generalizable to most patients seen clinically.
RCT trials often use average treatment effects from small, highly selective samples. Applying these results to the clinical population is problematic because of the lack of generalizability of these trials. Many clinical trials conducted in the US report on average treatment results, with some concluding that there is no treatment effect (Guyatt 2008). In essence, these studies are reporting on results for the “average” patient despite the variation in patient characteristics and outcomes seen in clinical practice (Kent 2018).
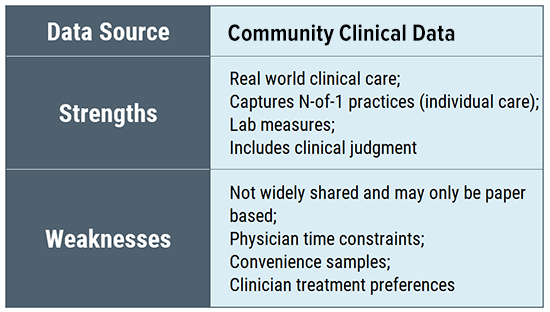
In contrast, patient registries reflect real-world behavior and practices and employ fewer inclusion and exclusion criteria (Dryer 2018). Studies with small sample sizes also preclude the type of subgroup analysis essential to identify treatment responders and only are able to detect large treatment effects (Kent 2018). Sample sizes in the thousands or tens of thousands are required to detect the small or moderate treatment effects that most patients would regard as meaningful (Guyatt 2008). Although a treatment may be clinically significant, the signal may not be apparent in a small study sample (National Academies of the Sciences, Engineering and Medicine 2017).
Because patient registries reflect real-world behavior and practices and employ fewer inclusion and exclusion criteria, they are more inclusive than randomized controlled trials and more generalizable to patients seen in clinical practice (Dryer 2018). They can also assess multiple treatment interventions used by clinicians in the real world and may reflect greater geographic diversity because they are internet based. Although patient registry samples are self-selected and rely on self-reported information, self-reported information is reported to improve accuracy of patient data and has been found to have acceptable levels of reliability when compared to medical chart information (Dryer 2018).
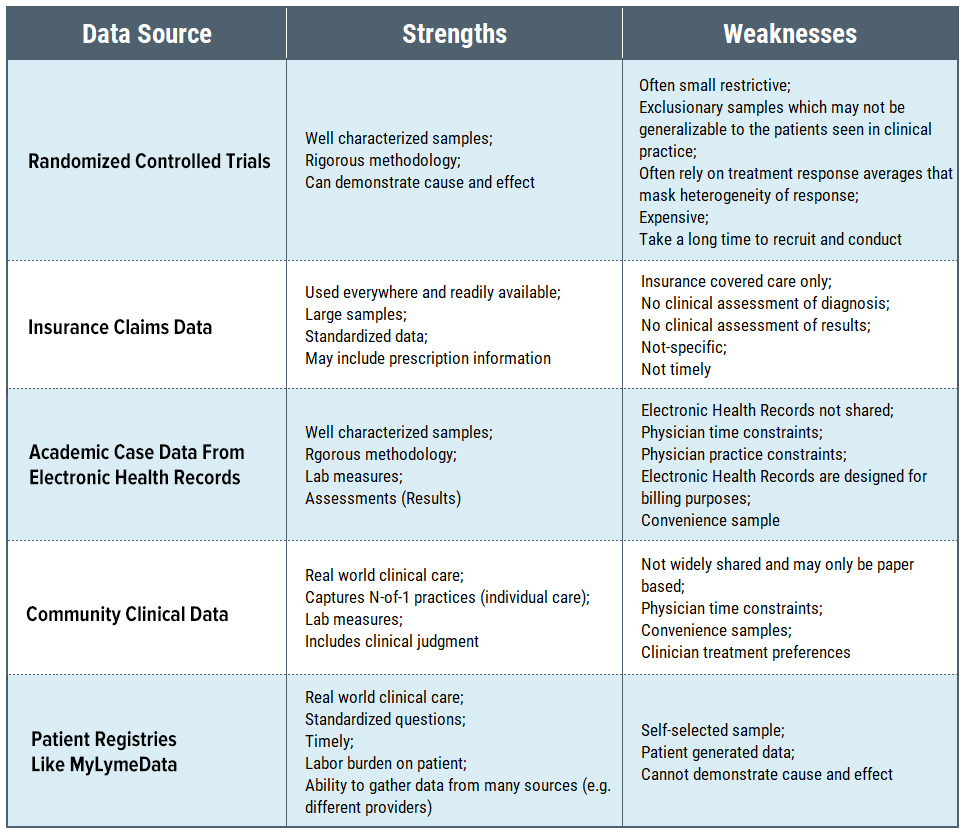
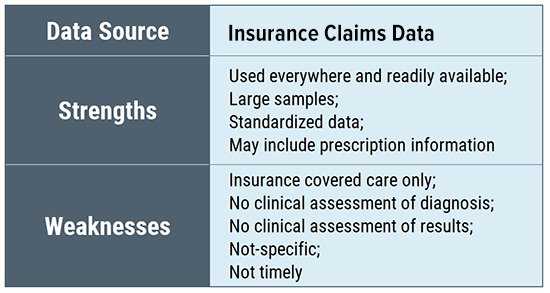
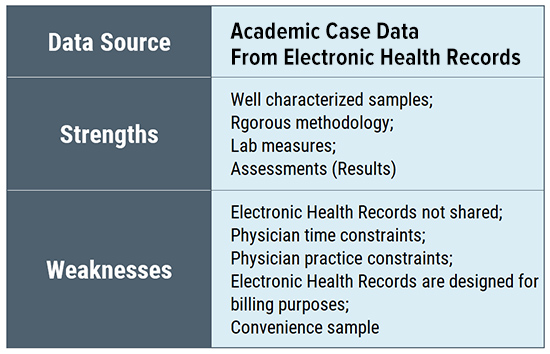
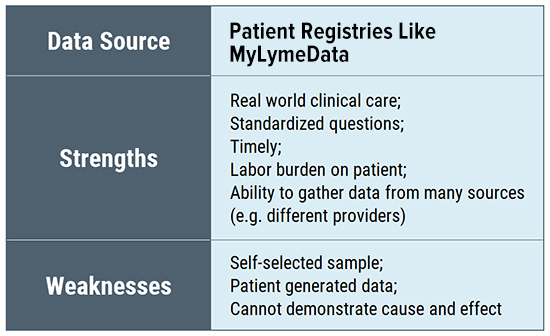
Similarly, convenience samples drawn from clinicians may have inherent sample bias reflecting the geographic location of the office as well as the treating style of the physician (and whether patients select to be treated by the physician). Insurance databases are broad but are limited to the data they collect and only include those whose treatment is covered by the insurance plan. (Many Lyme patients report that their physicians do not accept insurance.) Electronic health records may be limited by their intended utilitarian focus (billing claims).
The bottom line is that each form of evidence is vital in research despite the limitations inherent in each. Often ground truth is established when different forms of research evidence produce corroborating findings. Patient registries play a critical role in characterizing a disease, assessing real world treatments used in clinical settings, evaluating the effectiveness of different standards of care, and documenting treatment side effects. As such, they should be a central part of the research tool kit to improve patient care in Lyme disease.
- Bayliss, M.; Rendas-Baum, R.; White, M.K.; Maruish, M.; Bjorner, J.; Tunis, S.L. Health-related quality of life (HRQL) for individuals with self-reported chronic physical and/or mental health conditions: Panel survey of an adult sample in the united states. Health Qual. Life Outcomes 2012, 10, 154. https://pubmed.ncbi.nlm.nih.gov/23253258/
- Biau, D.J.; Kernéis, S.; Porcher, R. Statistics in brief: The importance of sample size in the planning and interpretation of medical research. Clin. Orthop. Relat. Res. 2008, 466, 2282–2288. https://pubmed.ncbi.nlm.nih.gov/18566874/
- Bytzer, P. Assessment of reflux symptom severity: Methodological options and their attributes. Gut 2004, 53 (Suppl. 4), iv28–iv34. https://gut.bmj.com/content/53/suppl_4/iv28
- Cohen, D.J.; Keller, S.R.; Hayes, G.R.; Dorr, D.A.; Ash, J.S.; Sittig, D.F. Integrating patient-generated health data into clinical care settings or clinical decision-making: Lessons learned from project healthdesign. JMIR Hum. Factors 2016, 3, e26. https://pubmed.ncbi.nlm.nih.gov/27760726/
- Collins, F. Patient led research: Citizen Scientists Take on the Challenge of Long Haul COVID-19. https://directorsblog.nih.gov/tag/patient-led-research/
- Delong, A.K.; Blossom, B.; Maloney, E.; Phillips, S.E. Antibiotic retreatment of Lyme disease in patients with persistent symptoms: A biostatistical review of randomized, placebo-controlled, clinical trials. Contemp. Clin. Trials 2012, 33, 1132–1142. https://pubmed.ncbi.nlm.nih.gov/22922244/
- Dreyer, N.; Franklin, P.; Haynes, K. Direct-to-patient registry and other patient-centric designs. In 21st Century Patient Registries: Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd ed. https://www.ncbi.nlm.nih.gov/books/NBK493817/
- Goswami, N.D.; Pfeiffer, C.D.; Horton, J.R.; Chiswell, K.; Tasneem, A.; Tsalik, E.L. The state of infectious diseases clinical trials: A systematic review of clinicaltrials. Gov. PLoS ONE 2013, 8, e77086. https://ohsu.pure.elsevier.com/en/publications/the-state-of-infectious-diseases-clinical-trials-a-systematic-rev
- Groft S. Patient Registries as a Prelude to Clinical Trials and Post-Approval Studies;. In Proceedings of Pediatric Devices for Rare Diseases Food and Drug Administration. White Oak, Maryland, January 8, 2014. https://www.fda.gov/media/87365/download
- Guyatt, G.H.; Mills, E.J.; Elbourne, D. In the era of systematic reviews, does the size of an individual trial still matter. PLoS Med. 2008, 5, e4. https://pubmed.ncbi.nlm.nih.gov/18177203/
- Howie, L.; Hirsch, B.; Locklear, T.; Abernethy, A.P. Assessing The Value Of Patient-Generated Data To Comparative Effectiveness Research. Health Aff. 2014, 33, 1220–1228. https://pubmed.ncbi.nlm.nih.gov/25006149/
- Johnson, L.; Shapiro, M.; Stricker, R.B.; Vendrow, J.; Haddock, J.; Needell, D. Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not? Healthcare (Basel) 2020, 8, doi: https://doi.org/10.3390/healthcare8040383.
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. In PeerJ, Wilke, C., Ed. 2014; Vol. 2, p e322. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3976119/
- Johnson, L.; Shapiro, M. Delayed Diagnosis Linked to Persistent Lyme Disease. (figshare). LymeMind Poster Session 2019, https://doi.org/10.6084/m9.figshare.9947105.v1
- Johnson, L.; Smalley, J. Engaging the Patient: Patient-Centered Research; Hall, K., Vogel, A., Croyle, R., Eds.; Springer: Switzerland, 2019; Vol. Chapter 10, pp. 507. https://www.researchgate.net/publication/337238133_Engaging_the_Patient_Patient-Centered_Research
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the Mask of Average Treatment Effects in Chronic Lyme Disease Research Using Big Data and Subgroup Analysis. Healthcare (Basel) 2018, 6, https://pubmed.ncbi.nlm.nih.gov/30322049/.
- Johnson, L.; Mervine, P.; Potter, M. New patient-powered research tool can be used to answer important questions about Lyme disease. In Proceedings of the Poster Presentation at the LDA/Columbia Conference, St. Paul, MN, USA, 16 October 2016.
- Johnson, L.; Aylward, A.; Stricker, R.B. Healthcare access and burden of care for patients with Lyme disease: a large United States survey. Health Policy 2011, 102, 64-71, doi:10.1016/j.healthpol.2011.05.007. https://pubmed.ncbi.nlm.nih.gov/21676482/
- Kent, D.M.; Nelson, J.; Dahabreh, I.J.; Rothwell, P.M.; Altman, D.G.; Hayward, R.A. Risk and treatment effect heterogeneity: Re-analysis of individual participant data from 32 large clinical trials. Int. J. Epidemiol. 2016, 45, 2075–2088 https://pubmed.ncbi.nlm.nih.gov/27375287/.
- Kravitz, R.L.; Duan, N.; Braslow, J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004, 82, 661–687 https://pubmed.ncbi.nlm.nih.gov/15595946/.
- National Academies of Sciences, Engineering, and Medicine. Enabling Precision Medicine: The Role of Genetics in Clinical Drug Development: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2017 https://pubmed.ncbi.nlm.nih.gov/28692241/.
- Peay, H.; Cope, E.; Horn, E.; Faulkner, M.; Carton, T.; O'Boyle, M.; Johnson, L. Building the Proper Foundation: Governance for Stakeholder-Engaged Research; Zimmerman, E., Ed. Sage: Virginia Commonwealth University, 2020; Vol. Chapter 17, pp. 477.
- Saver, J.L.; Kalafut, M. Combination Therapies and the Theoretical Limits of Evidence-Based Medicine. Neuroepidemiology 2001, 20, 57–64. https://pubmed.ncbi.nlm.nih.gov/11359071/
- Wood, W.A.; Bennett, A.V.; Basch, E. Emerging uses of patient generated health data in clinical research. Mol. Oncol. 2015, 9, 1018–1024. https://pubmed.ncbi.nlm.nih.gov/25248998/
If you are a patient who is not enrolled in MyLymeData, please enroll today. If you are a researcher who wants to collaborate with us, please contact me directly.
The MyLymeData Viz Blog is written by Lorraine Johnson, JD, MBA, who is the Chief Executive Officer of LymeDisease.org. You can contact her at lbjohnson@lymedisease.org. On Twitter, follow her @lymepolicywonk.