On April 28, I was honored to give a key note speech at the LymeX Innovation Accelerator, which was sponsored by the US Department of Health and Human Services and the Cohen Foundation. The Cohen Foundation has funded more research for Lyme disease than any other private organization. I spoke about why we need patient-partnered research to ensure that studies address the needs of patients living with the disease. I also talked about the role of the MyLymeData registry and the need to protect patient data so that it is used to benefit patients. You can watch my talk here:
A transcript of my talk follows:
LymeDisease.org is one of the oldest Lyme advocacy organizations in the nation — over 30 years old. It is also the largest and most trusted Lyme disease patient communications network. Our mission is to harness the power of tens of thousands of patients to improve patient care and accelerate the pace of Lyme disease research. We do this through communications, the MyLymeData patient registry and research platform, and science-based advocacy.
Today I am going to talk with you about what we need to do as a community to accelerate the pace of research and why I believe involving patients as research partners is essential to develop community trust and to ensure that research addresses questions that are important to patients and uses outcomes that patients value. Let me start by telling you a little about the registry.
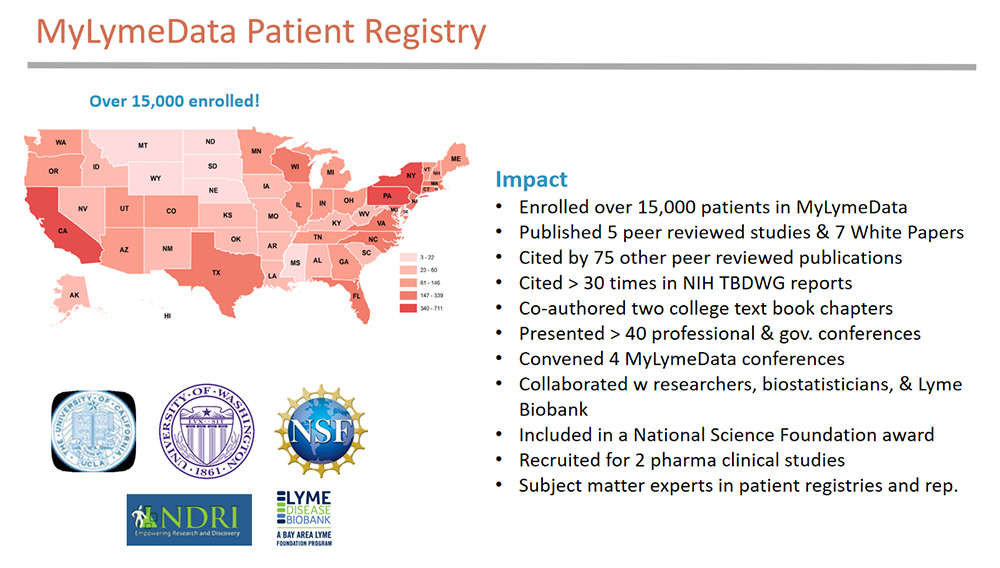
MyLymeData is a patient-led research registry that allows patients to pool their data to accelerate research using real world evidence. The registry uses the same technology and platform that serves NIH patient registries, it also uses government validated survey questions to the extent possible. Along the way we’ve formed collaborations with academic researchers, biostatisticians, and the Lyme Disease Biobank, a Bay Area Lyme Foundation Program, and have worked with two industry partners to recruit for clinical trials.
The MyLymeData patient registry was launched just five years ago. During this time, we have:
- Enrolled over 15,000 patients in MyLymeData
- Were included in a National Science Foundation award
- Published five peer reviewed studies that have been cited by 75 other publications
- Been cited over 30 times in NIH Tick-Borne Diseases Working Group reports and
- Co-authored two college text book chapters on patient registries and patient representation in healthcare
We are also recognized as subject matter experts in patient registries and patient representation and currently consult with PCORI through the University of Chicago NORC program.
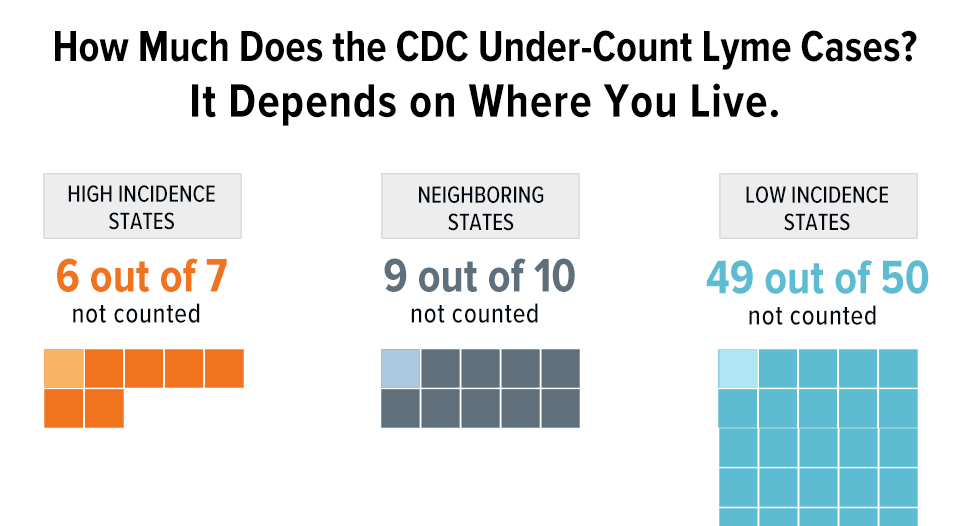
Lyme disease now has over 476,000 cases annually. But, according to research by Goswami, the number of clinical studies for Lyme disease trails behind leprosy — which has an incidence of less than 200 cases a year. So we need to think of Lyme disease as a research-disadvantaged disease that faces the same challenges and struggles that rare diseases face.
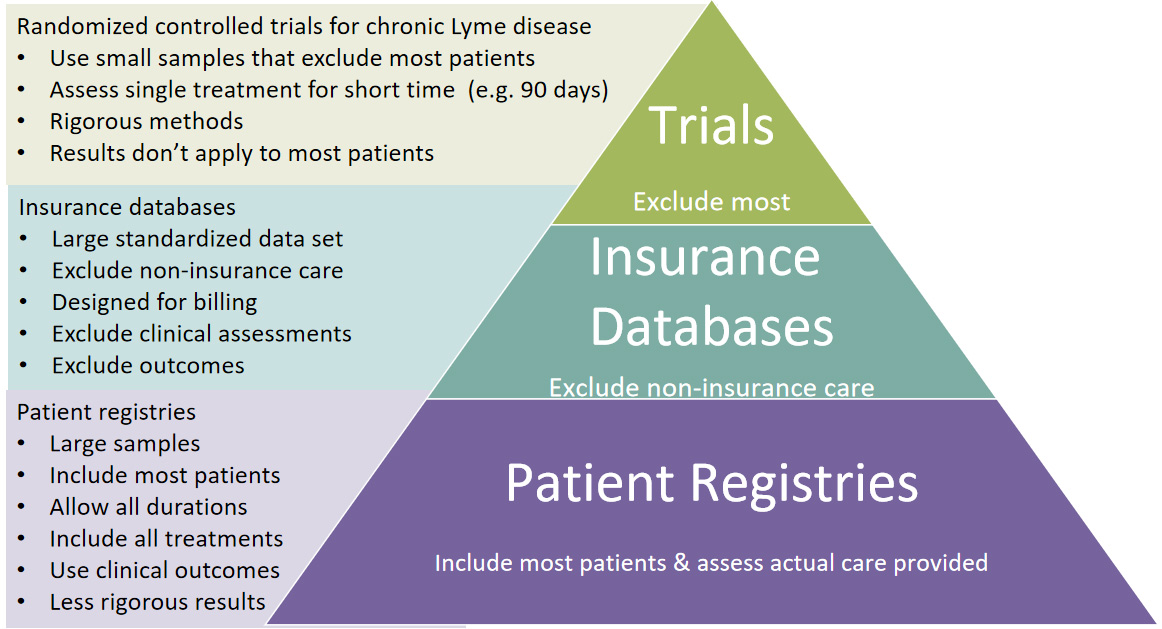
These diseases embrace innovative team research approaches that allow rapid knowledge generation to accelerate the pace of research. The research cycle illustrated here is derived from Groft’s work with rare diseases.
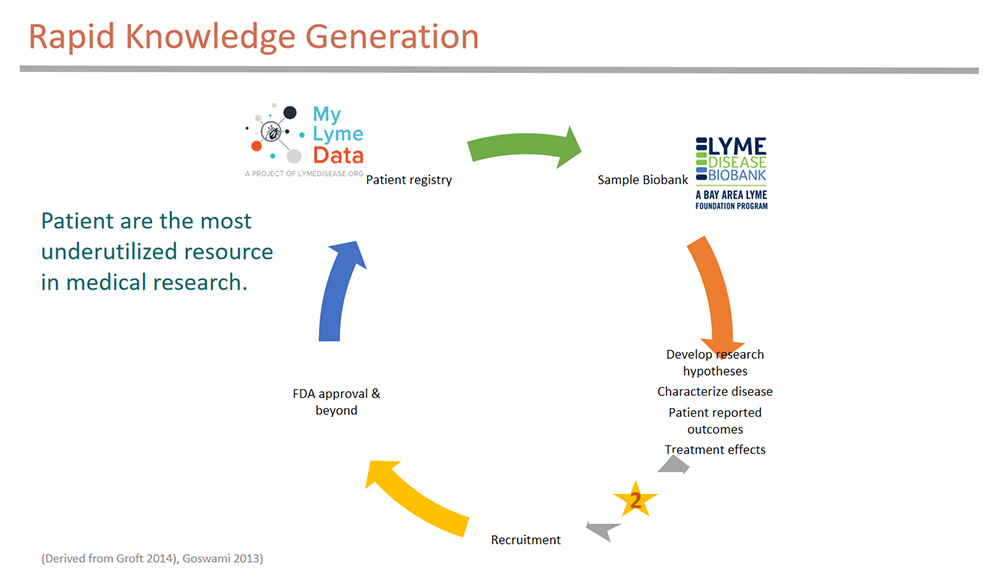
His model suggests forming a patient registry that links with a biorepository — we are collaborating with the Lyme Disease Biobank here. The registry helps develop research hypothesis — here we have published three peer-reviewed publications to better characterize the disease, assess patient reported outcomes, and analyze treatment effects among patient subgroups. The registry can then be used to help recruit patients. We have worked on recruiting for two clinical studies. What more do we need to do? To move forward, we have to embrace all forms of data and develop a more collaborative research effort approach in the community. We also need to address head on the data use trust issues that exist in the community.
Some of the challenges we face aren’t obvious, so I want to share with you a story about why MyLymeData withdrew from PCORnet, the big data project of the Patient Centered Research Outcomes Institute — also known as PCORI.
This story highlights issues surrounding patient trust in data use.

Shortly after PCORI was created by Congress to promote patient-centered research — I was selected to serve on their inaugural Patient Advisory Panel as well as on the Executive committee of its big data project PCORnet — which funded what was essentially an incubator for patient powered research networks — and included MyLymeData. This was an extraordinary opportunity to learn about developing patient registries from the best and the brightest in healthcare. I also served as the Chair of their patient council on data use, stigma, and privacy. and was included in the development of data-use policies — recommending patient-centered approaches.
But when the data-use policies were formalized by PCORI, the patient voice was largely ignored. The result was that patient community registries would not be able to control the use of their data. I knew this policy would not sit well with patients with persistent Lyme disease who are medically stigmatized and marginalized. And I knew that these patients would not enroll in MyLymeData if the use of their data could be used by others to further stigmatize the community. So we left PCORnet and set off to find funding for a registry where patients had control over data use.
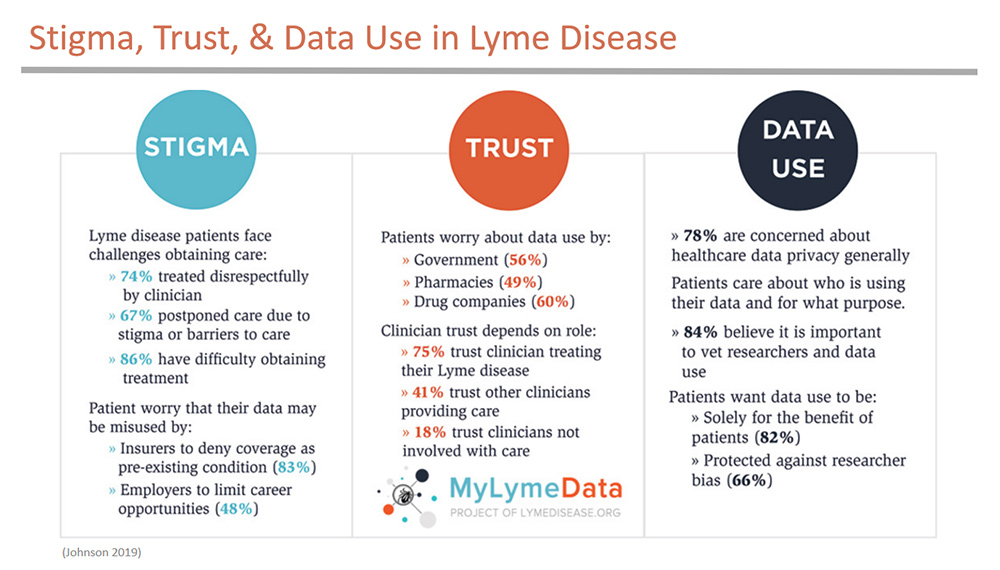
Here’s what I mean about patient data-use concerns. This slide shows results from our survey of over 1,900 patients enrolled in MyLymeData registry on stigma, trust, and data use.
When we asked patients about stigma, we learned that:
- 74% reported that they had been treated disrespectfully by clinician
- 67% postponed care due to stigma or barriers to care
- 86% have had difficulty obtaining treatment
Patients told us they were concerned about misuse of their data by insurers, employers, the government, and pharma. And they strongly believed that their data should be protected against researcher bias and should only be used for the benefit of patients.
Here’s a typical comment from one patient who took the survey:
“I think people are leery to share personal information because of how it is used by their job, insurance or government, which can all be untrustworthy. Sadly, people in our position have been discriminated against in one way or another.” — Lyme Survey Respondent
When patients go to see a doctor, they expect that physician to try to help them, if they go to the hospital they expect to be cared for — yet 74% report that they have been treated disrespectfully by clinicians. Patients tell us that they dread going to the hospital because their Lyme disease treatment will be terminated. They generally can’t find care through their customary sources of healthcare. Instead, they find care from clinicians who are often targeted by medical boards.
All this has created structural barriers to care which we detailed in Chapter 7 of the Tick-Borne Diseases Working Group this session. Patients with persistent Lyme disease have what Dr. Jennifer Freyd would call Betrayal Trauma — which occurs when the people or institutions on which a person depends for survival significantly violate that person’s trust or wellbeing. Many institutions involved with the Lyme community have earned patient distrust by — in one way or another — abandoning this patient population.
But trust is an essential component to creating a rapid-cycle research engine that uses big data.

Some people — including some researchers and some in government — believe that patient trust in data use is a secondary issue. But really it is the whole ball game. It’s why we pulled out of PCORnet.
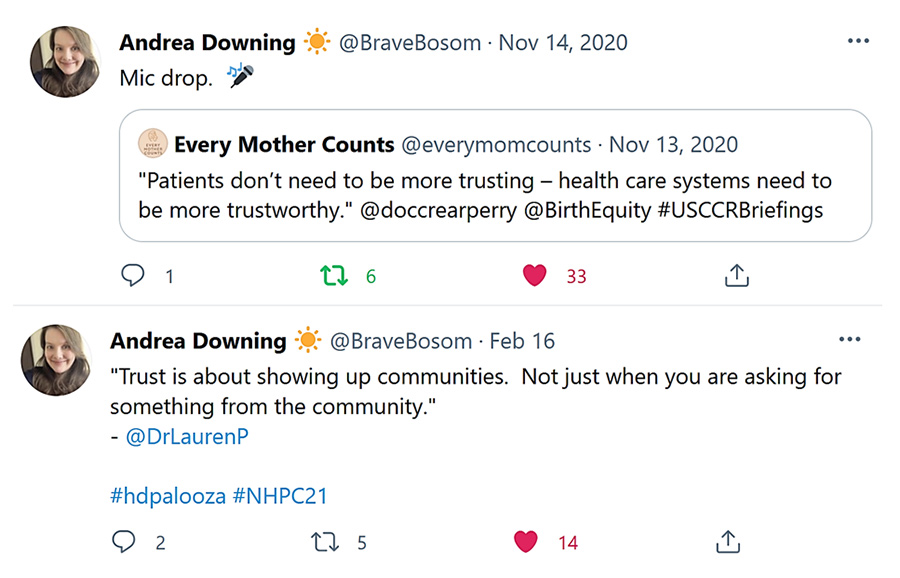
You might think “well patients just need to be more trusting.” But, “the real problem is that healthcare systems need to be more trustworthy.” — as Andrea Downing of BeLikeLight (@BraveBosom, Nov. 14, 2020), a patient data advocacy project, highlights. She emphasizes that “Trust is about showing up for communities. And not just when you are asking for something from the community” (@BraveBosom, February 16, 2021).
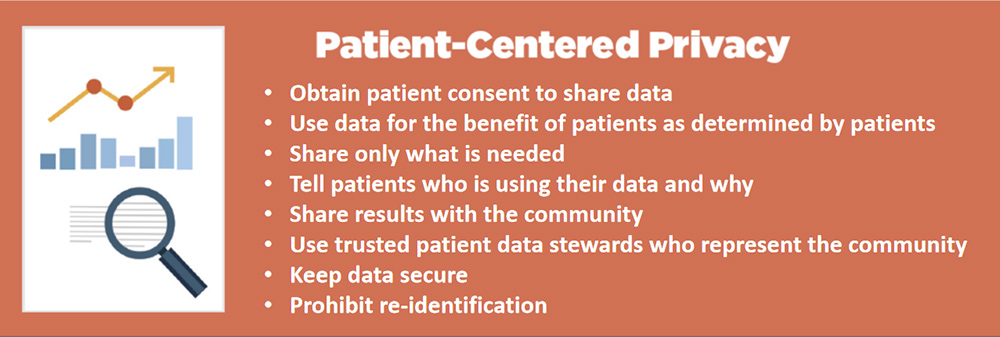
So what does it take for patients to trust that the use of their data will be used to benefit their interest?
Here’s what the MyLymeData patient registry does.
- We act as a trusted community data steward that safeguards the use of patients’ data for the benefit of patients.
- We have earned their trust over 30 years by consistently representing patient interests.
- We not only are patients ourselves, we also know what the community wants because we ask them through surveys.
- And we are accountable to the community.
If we get it wrong, patients walk with their feet — they can leave the registry at any time or never even sign up.
The very first question that patients want to know is what will the data be used for? There should be no surprises. When patients contribute their data to MyLymeData, that data should not be used to harm them.
The data steward principles that MyLymeData uses are easy to implement in a patient-led registry — although remember we did have to leave PCORnet and its funding in order to adopt them for the community. But other sources of big data like electronic health records or insurance data bases are not trusted data stewards for patients. And their use of patient data raises important issues. For example, patients may not have consented to engage in research — or may not know they have consented — or may not know what their data is being used for — and patients may not be able to take their data back if they disagree with its use. And, there is no way for patients to hold these data aggregators accountable to use the data for their benefit.
Finally, these sources of data do not and cannot represent patient interests — only patients authorized by and accountable to the community can do that. If we want to accelerate research, we need to use every tool at our disposal — all sources of data. But how can we do that in a way that is trustworthy to patients?
Dr. Harlan Krumholz, who Forbes refers to as “the most powerful doctor you never heard of,” was on the original board of PCORI and is a leading voice in research innovation.
Dr. Harlan Krumholz says that: “We need to restructure how traditional research is conducted to treat patients as research partners.”
He calls this Patient-Partnered Research.

This is research that sees patients as partners, not subjects. The researcher and the patients are involved in an on-going relationship — not one-off research projects. Research is conducted WITH patients not on them. Patients have agency over their data and results of research are returned to the community. This is where the Lyme community needs to be.
He goes on to say:
“Research is always better when the wisdom of those affected is recognized as central and operationalized as essential. And now is a time for all patients pursuing rapid knowledge generation speak with a common voice about how research is better when people facing the challenges are in a position to steer, have agency over their data,
are respected for their wisdom, and integrated seamlessly.”
Chicago Beyond, which funds research to change the lives of young people in Chicago, uses principles derived from Community Based Participatory Research methods. They require that researchers use an equity-based approach to research. Here’s how they define that:
“The creation of research should begin from a place of mutual understanding between community organizations, researchers, and funders. Those involved in the research design must recognize unintended bias to arrive at an authentic truth that does the most good for those being researched.” (Chicago Beyond 2019)
They also identify power dynamics that result in researchers not addressing the real problems the community faces.
They are saying that the point of research is to provide value to those being researched. They are also saying that you cannot arrive at authentic truth without active community participation. To earn that, researchers must be accountable to the communities they are researching. Success is measured by whether they in fact provide value to that community.
Now you might be thinking that I am simply saying that patients need a seat at the table. But that does not even begin to solve the problem — because patients are easily outnumbered and undervalued. As one Chicago Beyond researcher puts it:
People say “if you’re not at the table, you are on the menu. It’s more than that. It is how you are at the table. If you don’t decide what’s on the menu, if you are invited after the menu is set, you are still a guest. We as researchers get funded to be hosts. But in truth, the community should be the hosts, and researchers should be the guests.” (Chicago Beyond 2019)
But we have an opportunity to change that. Because of the HHS LymeX innovation project and the generous support of the Cohen Foundation, we have everyone here today needed to transform Lyme disease research into Patient-Partnered Research.
Ten years ago I would not have thought this community could do this. But today the moment has arrived and this community is now more than ready to engage as partners!
- Adams, W; Baumgarth, N.; Coyle, P.; Donta, S.; Fallon, B.; Johnson, L.; Jones, E.; Maloney, E.; Leiby, D.A.; Skare, J., et al. Report of the Pathogenesis, Transmission, and Treatment Subcommittee to the US HHS Tick-Borne Disease Working Group. 2018, https://www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/pathogenesis-transmission-2018-5-9/index.html.
- Chicago Beyond. Why Am I Always Being Research? White Paper 2019, https://chicagobeyond.org/researchequity
- Johnson, L.; Smalley, J. Engaging the Patient: Patient-Centered Research; Hall, K., Vogel, A., Croyle, R., Eds.; Springer: Switzerland, 2019; Vol. Chapter 10, pp. 507.
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. In PeerJ, Wilke, C., Ed. 2014; Vol. 2, p e322.
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the Mask of Average Treatment Effects in Chronic Lyme Disease Research Using Big Data and Subgroup Analysis. Healthcare (Basel) 2018, 6, doi:10.3390/healthcare6040124.
- Johnson, L.; Aylward, A.; Stricker, R.B. Healthcare access and burden of care for patients with Lyme disease: a large United States survey. Health Policy 2011, 102, 64-71, doi:10.1016/j.healthpol.2011.05.007.
- Johnson, L. (2019): Stigma and Privacy in Lyme Disease [White Paper]. https://doi.org/10.6084/m9.figshare.7704167.
- Johnson, L. (2019): 2019 Chart Book – MyLymeData Registry. (Phase 1 April 27, 2017. Sample 3,903). figshare. White Paper. https://doi.org/10.6084/m9.figshare.7849244.
- Johnson, L.; Maloney, E.; P, S.; Bunnell, R.; DuLaney, M.; Fearn, D.; Statlender, S. 2020 Training, Education, Access to Care, and Reimbursement Subcommittee Report to the US HHS Tick-Borne Disease Working Group. 2020, www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/training-education-access-to-care-and-reimbursement-subcomm-2020/index.html
- Peay, H.; Cope, E.; Horn, E.; Faulkner, M.; Carton, T.; O'Boyle, M.; Johnson, L. Building the Proper Foundation: Governance for Stakeholder-Engaged Research; Zimmerman, E., Ed. Sage: Virginia Commonwealth University, 2020; Vol. Chapter 17, pp. 477.
- Fleurence, R.L.; Beal, A.C.; Sheridan, S.E.; Johnson, L.B.; Selby, J.V. Patient-Powered Research Networks Aim To Improve Patient Care And Health Research. Health Affairs 2014, 33, 1212-1219, doi:10.1377/hlthaff.2014.0113 content.healthaffairs.org/content/33/7/1212.abstract
- Goswami, N.D.; Pfeiffer, C.D.; Horton, J.R.; Chiswell, K.; Tasneem, A.; Tsalik, E.L. The state of infectious diseases clinical trials: a systematic review of ClinicalTrials.gov. PLoS ONE 2013, 8, e77086, doi:10.1371/journal.pone.0077086.
- Groft S. Patient Registries as a Prelude to Clinical Trials and Post-Approval Studies;. In Proceedings of Pediatric Devices for Rare Diseases Food and Drug Administration White Oak, Maryland, January 8, 2014.
- Vendrow, J.; Haddock, J.; Needell, D.; Johnson, L. Feature Selection from Lyme Disease Patient Survey Using Machine Learning. Algorithms 2020, 13, 334, https://www.mdpi.com/1999-4893/13/12/334
If you are a patient who is not enrolled in MyLymeData, please enroll today. If you are a researcher who wants to collaborate with us, please contact me directly.
The MyLymeData Viz Blog is written by Lorraine Johnson, JD, MBA, who is the Chief Executive Officer of LymeDisease.org. You can contact her at lbjohnson@lymedisease.org. On Twitter, follow her @lymepolicywonk.